Back by popular demand” always seemed a self-congratulatory phrase, but in this case it really is the case that I’m reviving this blog because so many people have said they found it helpful. I’m touched by that, and also grateful to be given the motivation to continue pursuing this endlessly fascinating topic. So here we go.
One of the things that led me to let the blog languish was that there were so many other areas in which I have been striving to deepen my knowledge over the past several years. Happily, at least one of these remains relevant to the topic at hand: I have been delving deeper into the thickets of molecular and cell biology for my latest book, How Life Works, which is published in the autumn/fall of 2023. Among many other things, this looks at issues such as the roles of disordered proteins and of biomolecular condensates, in which issues of solvation are clearly important.
As if to underscore that, I am kicking off this post with a fascinating paper in Nature by Benjamin Schuler at Zurich and colleagues [N. Galvanetto et al., Nature 10.1038/s41586-023-06329-5; 2023], which looks at biomolecular dynamics within such a dense condensate. I probably don’t need now to say much about the importance of condensates in cell biology; as Schuler and colleagues say, these dense but loose associations of proteins and nucleic acids “play a key role in cellular processes operating at different scales, such as ribosome assembly, RNA splicing, stress response, mitosis and chromatin organization, and they are involved in a range of diseases” (and also, I’d add, in gene regulation). Their significance was confirmed by the fact that Tony Hyman and Cliff Brangwynne, who did much of the early work to bring them to wider attention, were awarded last year’s Breakthrough Prize in the life sciences. As Schuler et al. say, intrinsically disordered proteins seem often to play a key part in the formation of condensates, thanks to their rather promiscuous binding capacity.
The dense fluid of these phase-separated blobs can have a greatly enhanced local concentration of constituents relative to the bulk cytoplasm, and indeed this is a central feature of their function: they help to concentrate particular biomolecular species to enable repeated, low-affinity encounters of the kind that seem important for e.g. gene regulation or splicing. That density, however, results in a water viscosity that can be several orders of magnitude greater than it is in the bulk. Schuler and colleagues use single-molecule spectroscopy and MD simulations to examine the consequences of this for the dynamics of proteins. They study in vitro proxies for in vivo condensates, consisting of droplets (coacervates – I’m interested to see the return of that word from the days of the “prebiotic” speculations of Sidney Fox!) made from two highly and oppositely charged IDPs, both of which are known to influence chromatin condensation and transcriptional regulation.
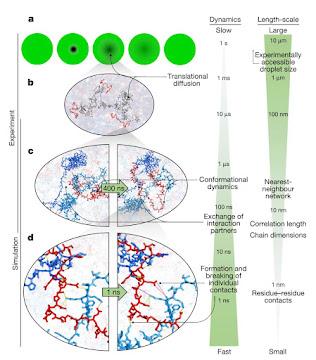
The result is that, despite a 300-fold increase in water viscosity in the droplets and the formation of a dense network of indiscriminately associated proteins, leading to drastically slowed translational motion, the local conformational dynamics of the chains that influence residue-residue contacts remain fast, happening on pico- to nanosecond timescales. The conclusions are nicely summarized in this image:
As the authors say: “The behaviour we observe is an example of the subtle balance of
intermolecular interactions in biomolecular phase separation. On the one hand, the interactions must be strong enough for the formation of stable condensates; on the other hand, they need to be sufficiently weak to enable translational diffusion and liquid-like dynamics within the dense phase and molecular exchange across the phase boundary— processes that are essential for function, such as biochemical reactions occurring in condensates.” I have to say that this doesn’t entirely surprise me, as it seems to reflect in a more extreme way what seems to happen in cells more generally: the cytoplasm can look somewhat gel-like at mesoscales, and translational diffusion can be anomalous, while at the molecular scale dynamics are not so different from those in dilute solution. Of course, the usual caveats about in vitro studies – which have been stressed in particular for studies of condensates (see e.g here) – apply too. But this is a really nice study, which to my mind illustrates the possibility of a separation of dynamical timescales that makes life possible: the benefits of forming condensates do not need to come at the expense of compromising the local dynamics needed for biomolecular function.
Needless to say, I have from time to time seen some wonderful papers touching on water in biology come and go in the time between my last blog and now, and I fear these are lost now to being documented here. But there are a few recent ones that I still have to hand. There has been a particularly vigorous debate in the past year or two on the issue of the putative liquid-liquid phase transition in pure water. The evidence for this continues to accumulate, and looks now to be rather strong – although that clinching proof remains elusive. This paper [Science 359, 1127; 2018] by Sander Woutersen in Amsterdam – including the late and much missed Austen Angell – reported a direct sighting of such a transition in an aqueous solution of hydrazinium trifluoroacetate, which could be deeply supercooled to 140 K. And work by Yoshiharu Suzuki in Tsukuba adds to that suggestive evidence with a study [PNAS 119, e2113411119; 2022] of supercooled aqueous trehalose, which reports a direct signature of a first-order liquid-liquid transition. You can see some responses to the work here. Meanwhile, Nguyen Vinh and colleagues at Virginia Tech have performed terahertz measurements of water over a wide temperature range, down to the deeply supercooled state, which they say can be interpreted it terms of two liquid forms with different transition temperatures.
I am (not so?) secretly delighted that my PhD supervisor Bob Evans at Bristol decided some time ago to delve into water phase behaviour, having warned me (wisely!) to steer clear of any liquid so anomalous. Here [J. Chem. Phys. 158, 034508; 2023] Bob, Mary Coe and Nigel Wilding suggest that the well known density depletion and enhanced fluctuations evident in water near hydrophobic surfaces be interpreted as the remnant of a critical surface (drying, or as some call it, dewetting) phase transition. In other words – and this seems to me quite significant – there is in a sense nothing unusual about water in this respect, and no need to invoke any specialness about its liquid-state structure. Rather, this is well-understood statistical physics at work. I notice that this was the topic of Mary’s 2021 PhD thesis: “Hydrophobicity across length scales: The role of surface criticality” – which I’d love to see.
I can’t touch on that topic without remembering that for my last post in January 2018, I seem not yet to have caught up with the news of the death in April 2017 of David Chandler, a giant in the field of liquid-state theory, as well as statistical mechanics more broadly. David’s work with John Weeks and Ka Lum on the hydrophobic interaction [JPCB 103, 4570; 1999] has of course been one of the most influential contributions to this topic since it was published in 1999. David’s work has certainly been a huge influence on my thinking in this field – and indeed that influence went right back to my work with Bob in the mid-1980s. David was always generous but rigorous, and phenomenally insightful, and his absence is deeply felt.
Francesco Paesani of UCSD has let me know about the new water potential he and his colleagues have developed from first-principles quantum simulations, which is able to capture the phase diagram very realistically [Bore & Paesani, Nat. Commun. 14, 3349; 2023] – see also the improvements in Zhu et al., J. Chem. Theory Comput. 19, 3551-3566; 2023]. Francesco has already been applying it to a variety of real-world problems, including the hydration of a simple model of the protein backbone (N-methylacetamide) [Zhou et al., J. Chem. Theory Comput. 19, 4308; 2023].
I just noticed this interesting article [A. Katsnelson, ACS Cent. Sci. 10.1021/acscentsci.3c00803; 2023] on “artificial saliva” and synthetic mucins. I knew nothing about such work, which sound like rich ground for exploring hydration issues.
I can’t end this first post without mentioning the ongoing story of “life (or not) in the clouds of Venus”. This intriguing idea was first put forward by Sara Seager and her colleagues following the apparent identification [J. S. Greaves et al., Nat. Astron. 5, 655; 2021] of phosphine – for which no abiotic natural source is known – in the Venusian atmosphere. Much of the immediate debate (aside from that about how secure the signature of phosphine really was, a discussion that is still ongoing) centred on the extreme acidity of the cloud droplets. But I was more concerned about how low the water activity in the droplets must be – we know that no life on Earth has been seen for water activities below 0.585, a fact that I am sure is related to the minimal hydration requirements for functional biomolecules. When I mentioned this to microbiologist John Hallsworth at Queens in Belfast, he convened a team of experts who made calculations to estimate the water activity in the Venusian clouds and a variety of other planetary environments, revealing that on Venus this is indeed orders of magnitude too low to permit terrestrial-type life given what we currently know about it [J. E. Hallsworth et al., Nat. Astron. 5, 665; 2021]. Sara and her coworkers have now suggested [W. Bains et al., Astrobiol. 10.1089/ast.2022.0113; 2023] possible ways around this problem, which in the end amount to having to posit life unlike that on Earth – a possibility we recognized in our paper. To my mind, this could usefully sharpen the discussion about what “habitable” can and should mean in astrobiology, as I explore here. I welcome anything that prompts deeper, quantitative thinking about the conditions needed to support something we might reasonably call life in extraterrestrial environments. But I would argue that such discussions need to go beyond “well, X can also act as a hydrogen-bonding solvent”, and should take into consideration just how subtle water’s roles are in terrestrial biochemistry, and how much the “specialness” of water might matter in this regard. Given the richness of emerging knowledge about extraterrestrial environments, and the prospects of much more to come through planned missions (e.g. to Enceladus and Titan) and JWST observations, I’d love to see this issue engaged with head-on by e.g. ESA or NASA.
Well, there we are: I’m open for business again. So do feel free to send things my way for inclusion; I’d be delighted to hear about them.